FS Input


the functional specification database
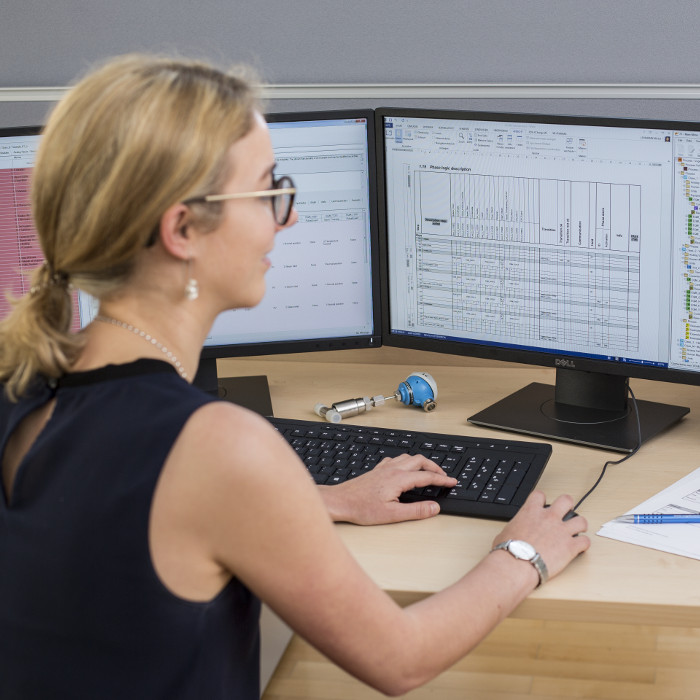
FS INPUT® is a database tool for the creation of functional specification documents for process automation of batch operated biotechnological plants. FS INPUT® was developed to meet the high standards of GMP regulation in life science industry. It enables the user to generate functional specification documents in a standardized and consistent way. The flexible and modular structure of FS INPUT® follows the guidelines of ANSI/ISA-88 and allows the execution of various automation concepts used in large and complex projects.
As one of the world’s leading suppliers of engineering for biotechnological plants, Vogelbusch Biopharma developed FS INPUT® to meet the high project requirements in terms of quality, timeline and documentation standards. Especially in the field of automation, the use of standard office software becomes impractical once a project exceeds a certain size and complexity. The need to guarantee consistent quality and to avoid repetitive work motivated us to develop FS INPUT®.
tried and tested for more than 10 years
FS INPUT® has been used in many projects with various automation systems for more than 10 years. Continuous improvement and optimization resulted in a lean and efficient software with a focus on:
- Meeting the requirements of complex and large scale projects with hundreds of documents and thousands of automated valves
- Being independent of the selected automation system and supplier
- Supporting the total life cycle of functional specification documents - documents can be used for software coding, as test documents for qualification and for documentation of process optimization
- Saving time and costs by avoiding unnecessary tasks and workarounds
central data and revision management
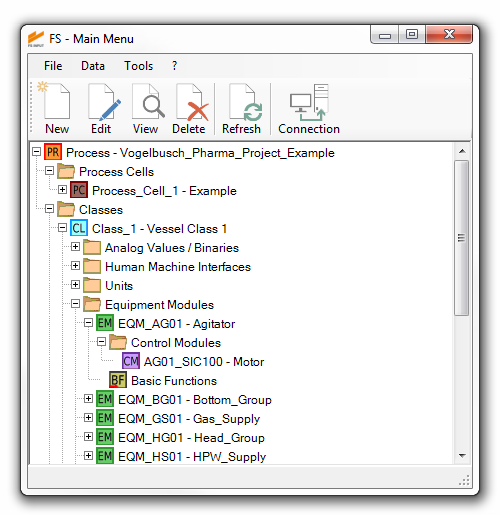
In order to provide the highest level of data integrity, FS INPUT® stores all information in a central database. Efficiency is increased and workarounds are reduced, as data entry and modification only need to be made once and the current state of the project is accessible to all users at the same time.
We know that version control is an essential feature in engineering. In FS INPUT® each document has a unique revision number and editing state (e.g. “for review”, “for approval”…) as well as a detailed revision history. Earlier revisions are saved and can be viewed but no longer be manipulated in order to maintain integrity and traceability.
You can track all changes by a revision compare function. Differences between an earlier version and the current state of a functional specification can be viewed and compiled into a document.
customized document format
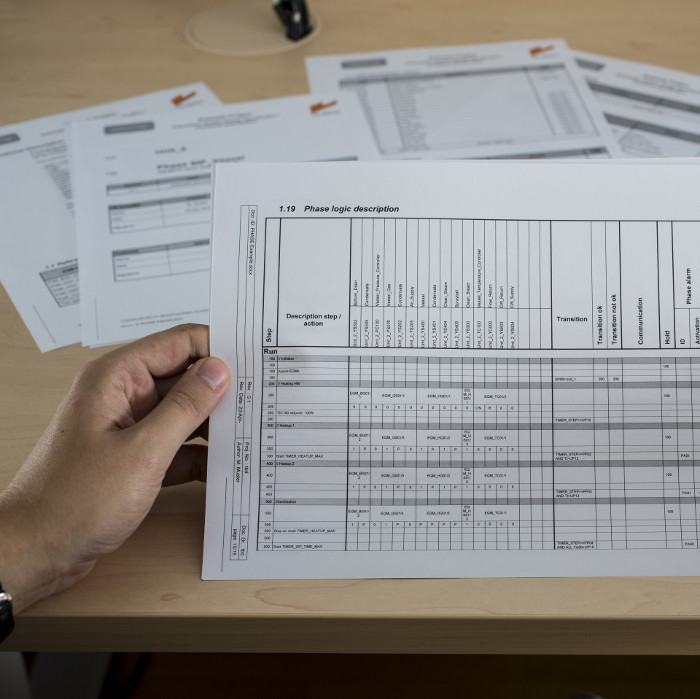
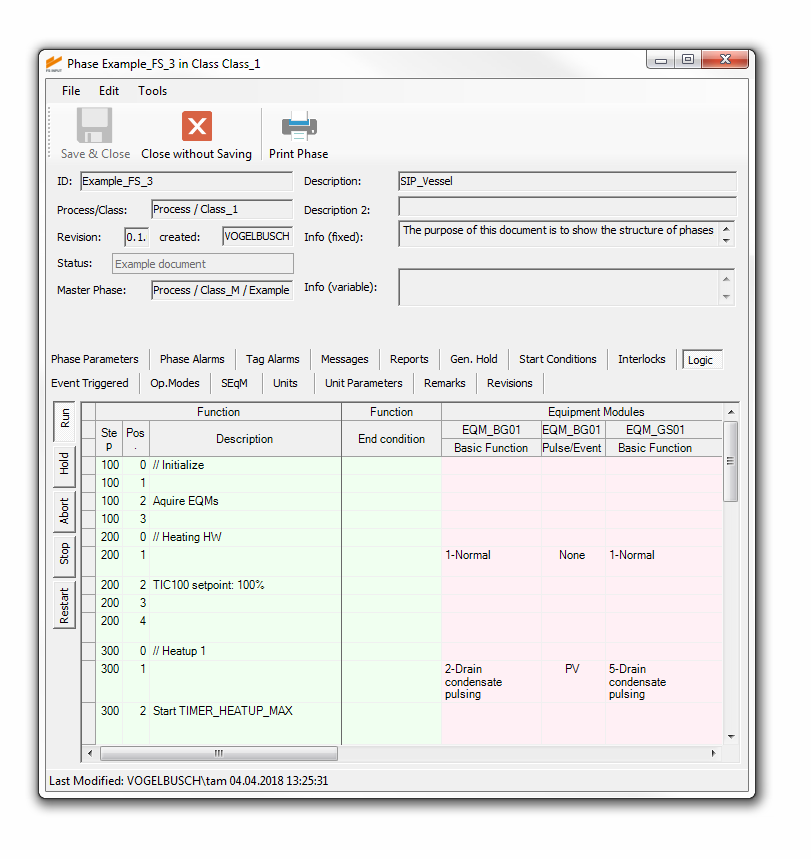
All information and details allocated to a certain process step (e.g. transfer, sterilization or cultivation) is edited via tailored user interfaces. FS INPUT® can compile the information into documents which can be used as functional specifications for software coding, qualification and process optimization.
As the valve matrix is one of the most important parts of a functional specification document, we optimized the printout format. A poster-sized valve matrix is split into folder-sized pages for easy handling. Intelligent spacing and pagination make sure that the valve matrix keeps its consistency.
In addition to the functional specification documents, a variety of supplementary spreadsheets can be compiled in order to support efficient software coding.
customer support at all levels

In the development of FS INPUT® a lot of effort was put into keeping it “easy to use”. Nevertheless the users of an engineering software need to become familiar with the tool as quickly as possible. Therefore, we offer training packages that cover all functions of FS INPUT® and enable you to achieve the best results right from the start.
With the experience from numerous projects our experts can help you to set up a project in FS INPUT®. Consulting and hands-on support is provided for either creating a new database or importing an existing project.
Also if you are already experienced in using FS INPUT® in your daily project work, you can rely on our assistance and benefit from a ticket-based online support system as well as an online knowledge database.
benefits
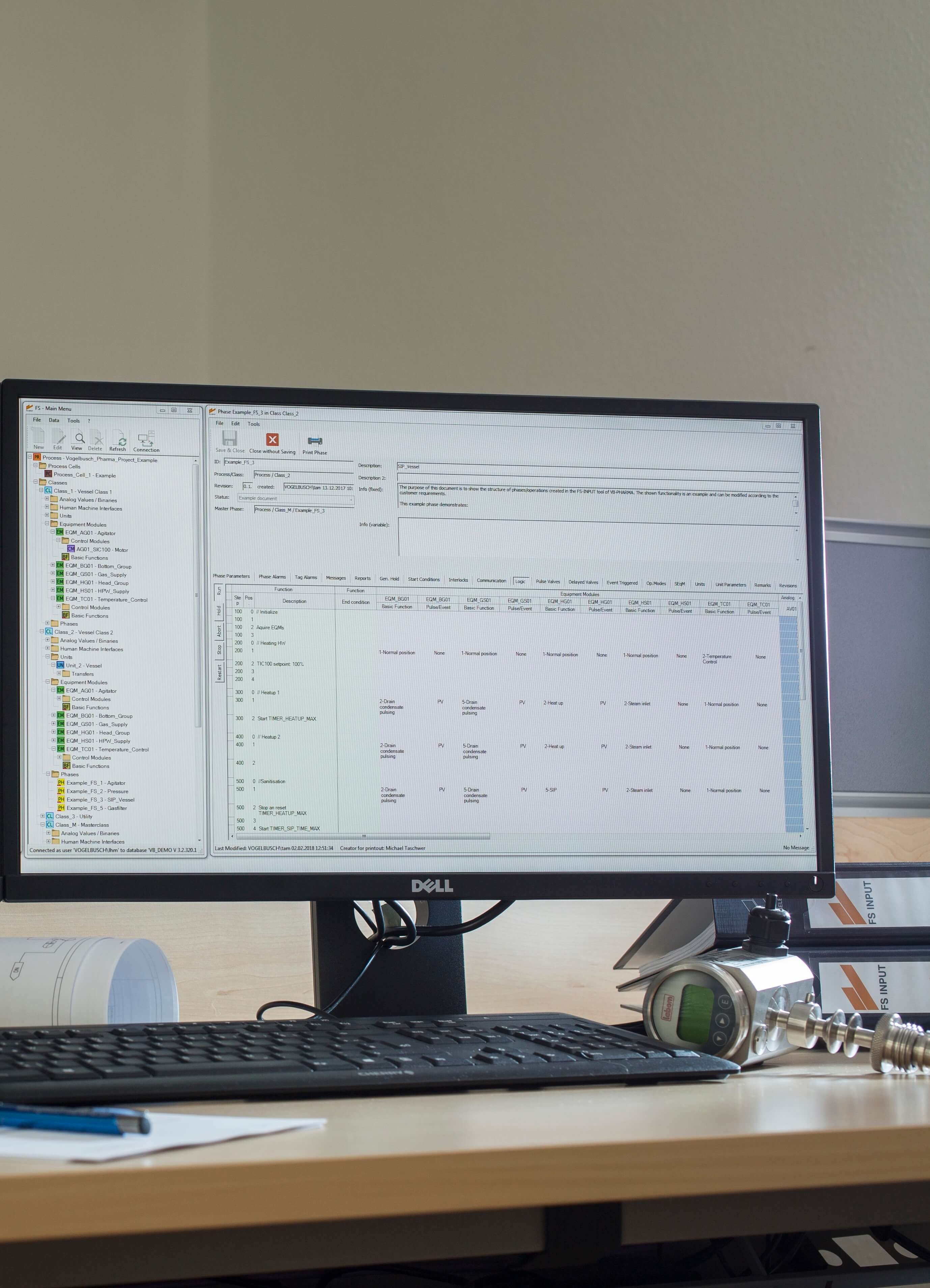
- Generation of functional specification documents in a standardized and consistent way for projects in a GMP environment
- Flexible and modular structure following the current guidelines of ANSI/ISA-88
- Suitable for complex and large scale projects
- Significant reduction of effort and high level of consistency due to master / reference system and class-based approach for physical model of the plant
- Central data and revision management
- Support the total life cycle of functional specification documents - documents can be used for software coding, qualification and process optimization
- Reduction of effort for the subsequent generation of functional design specifications due to the high standard of functional specification documents generated in FS INPUT®
- Independent of the selected automation system
- Optimized for process automation of batch operated biotechnological plants
contact
VOGELBUSCH BIOPHARMA GmbH
![]() Blechturmgasse 11
Blechturmgasse 11
1051 Wien | Österreich
![]() +43 1 54 661-102
+43 1 54 661-102
![]() +43 1 54 661-100
+43 1 54 661-100
![]() fsinput@vogelbusch-biopharma.com
fsinput@vogelbusch-biopharma.com![]() fsinput.vogelbusch-biopharma.com
fsinput.vogelbusch-biopharma.com

